Best wishes for 2019 from Imavita’s team
All the team of Imavita wishes to all collaborators, colleagues, customers and suppliers and their families, an excellent and happy new year 2019!

All the team of Imavita wishes to all collaborators, colleagues, customers and suppliers and their families, an excellent and happy new year 2019!

IMAVITA S.A.S., an innovating technology company providing services in preclinical models of human pathologies associated with imaging and image analysis, announces its participation in the collaborative project Pi-R2 / Innovative Technical Radiofluorination Platform for Radiochemistry and Radiopharmacy of Fluor-18.
This 3-year project will focus on the fields of oncology, cardiovascular and neurology, for which 4 work teams have been created:
IMAVITA will actively participate in this project by providing technical and scientific expertise and relevant preclinical models of cancer, atherosclerosis and Alzheimer’s disease.
This project will allow IMAVITA to develop new animal models, access custom and innovative radio tagging skills, explore existing preclinical models in PET/CT imaging and develop new translational services in preclinical and clinical imaging.
Partners participating in the Pi-R2 project:
ToNIC (Toulouse NeuroImaging Center) http://tonic.inserm.fr
LCC CNRS https://www.lcc-toulouse.fr/
CREFRE https://www.iuct-oncopole.fr/le-crefre
CRCT https://www.crct-inserm.fr/
I2MC http://www.i2mc.inserm.fr/
Zionexa http://zionexa.com
Imavita https://imavita.com

IMAVITA S.A.S., société de services et d’innovation dans les modèles précliniques de pathologies humaines associées à l’imagerie et l’analyse d’images, annonce sa participation au projet collaboratif PiR2 / Plateau Technique innovant de Radiochimie et de Radiopharmacie du Fluor-18.
Ce projet de 3 ans sera axé dans les domaines de l’oncologie, cardio-vasculaire et neurologie et pour lesquels 4 équipes de travail ont été créées :
1/ Synthèse, purification et analyse structurale de précurseurs de radio-marquage avec le Fluor 18 et mise au point des diverses synthèses de molécules radio-fluorées,
2/ Production des molécules radio-marquées pour injection dans des modèles pré-cliniques,
3/ Développement et mise en place des modèles animaux pathologiques, imagerie PET/CT et analyse quantitative des données d’imagerie pour validation du ciblage thérapeutique,
4/ Production en qualité pharmaceutique et utilisation des productions de produits radio-marqués dans le cadre d’essais cliniques
La société IMAVITA participera activement à ce projet par la mise à disposition de compétences techniques et scientifiques et la fourniture de modèles précliniques pertinents de cancer, d’athérosclérose et de maladie d’Alzheimer.
Ce projet permettra à IMAVITA le développement de nouveaux modèles animaux, l’accès à des compétences de radio-marquage à façon et innovants, l’exploration en imagerie PET/CT de modèles précliniques existants et de nouveaux services translationnels en imagerie préclinique et clinique.
Partenaires participant au projet PiR2:
ToNIC (Toulouse NeuroImaging Center) http://tonic.inserm.fr
LCC CNRS https://www.lcc-toulouse.fr/
CREFRE https://www.iuct-oncopole.fr/le-crefre
CRCT https://www.crct-inserm.fr/
I2MC http://www.i2mc.inserm.fr/
Zionexa http://zionexa.com
Imavita https://imavita.com

Imavita released the use of OCT (Optical Coherence Tomography) for in vivo imaging in the following applications:
This imaging technique is available for in vivo (isofluran anesthesia) and/or ex vivo imaging and permits to accelerate evaluation of efficacy of compounds during course of projects or on sample collection.
Image example (atopic dermatitis model / ear):
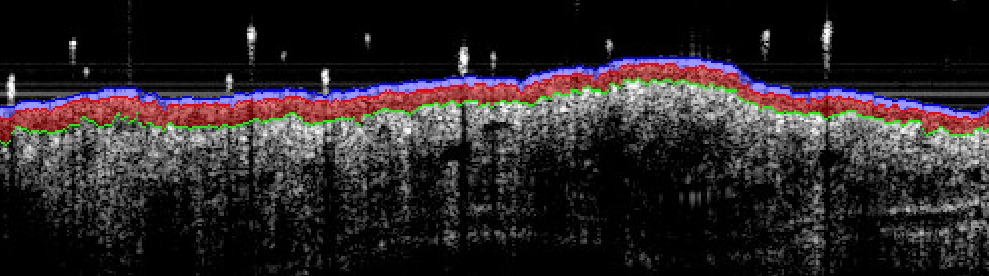
Imavita developed a quantification algorithm to permit measurement of epidermis thickness immediatley after image capture. Correlation and validation of this imaging biomarker was fully performed in comparison to histology.
Release of in vivo OCT imaging in preclinical models was performed on November 2018.
Imavita is proud to announce the acquisition of new Optical Coherence Tomography apparatus to develop Innovating imaging of dermatology models.
Since september 2017, Imavita is working on this innovative imaging system (OQ Labscope / Lumedica) which will permit to generate non-invasive, surface tissue quantitative data for pre-clinical models in place at Imavita in Applications of interest, principally in the area of dermatology.
The benefits of this in vivo imaging modality are:
For more informations, do not hesitate to contact us.
Since January 2nd 2017, Imavita laboratories address moved to Canal Biotech 1, 3 rue des Satellites, 31400 Toulouse, France.
This address will be the same for headquarters.
The labs will approximate the team to the in-life / in vivo facilities in which experiments are performed.
Canal Biotech is a public building (Toulouse Metropole / SEMIDIAS) for company housing dedicated to life sciences.

Team is growing! Imavita is proud to announce the recent recruitment of Elisabeth Bonnard and Jeanne Chapel.
Team is growing!
Imavita is proud to announce the acquisition of new Fluorescence Molecular Tomography imaging apparatus for the evaluation of its models.
Since october 2016, a Fluorescence Tomography system was acquired (FMT2500, Perkin Elmer). The FMT2500 fluorescence tomography imaging system provides the greatest utility of the FMT Systems with the ability to quantitate up to 2 fluorophores simultaneously. It comes with 2 excitation laser channels (680 & 750 nm).
This imaging modality will permit to generate non-invasive, deep tissue quantitative data for pre-clinical models in place at Imavita in Applications of interest.
The benefits of this in vivo imaging modality are:
For more informations, do not hesitate to contact us.

Imavita has Research Tax Credit agreement / CIR (“Crédit Impôt Recherche“) for years 2016 to 2020 (obtained on April 22th 2016 / download pdf file of Research Tax Credit Accreditation).
This agreement, obtained from the french authorities, permits to the clients working with Imavita to refund R&D spendings to their corporate taxes.
See the following links for more informations on CIR / Research Tax Credit: MESR (Ministère de l’Enseignement Supérieur et de la Recherche)
Summary on Research Tax Credit
All companies incurring R&D expenses are eligible to receive France’s research tax credit, regardless of their size, business sector and nationality.

The tax credit covers 30% of all R&D expenses and covers all R&D spending: Salaries, social security contributions, amortization and depreciation allowances, operating costs, subcontracting, patents and monitoring.
The scope of SME spending eligible for the research tax credit has been extended as of 2013 to cover innovation expenses arising from designing prototypes, as well as pilot plants for new products. These expenses are now included in the research tax credit base, at a rate of 20%.
For income tax purposes, the definition of a qualified activity for the Research & Experimentation (R&E) Tax Credit or the research and development (R&D) Tax Credit includes not only the development of new products, but also improvements to products, processes, techniques, formulas, patents, and software applications.
The french authorities approved on September 2013 Imavita as an Innovative New Company (“Jeune Entreprise Innovante / JEI”) based on its ability and capacity of performing R&D projects.