Imavita | Partner in preclinical models research and imaging solutions
Choose the best preclinical approaches to:
- Better mimic the complexity of human disease mechanisms
- Enhance the success of drug R&D
At Imavita, we are an innovative preclinical CRO, specializing in advanced imaging and biomarker analysis.
We help pharma, biotech, and research labs assess the efficacy, disposition and safety of new therapies with
precise, non-invasive tools across in vivo, in vitro,
and ex vivo models.
Preclinical services to evaluate
Drug disposition and efficacy &Non invasive and quantitative imaging techniques
Discover our customized in vivo models designed to assess
efficacy/pharmacodynamics (PD),
drug disposition/pharmacokinetics (PK),
and non-GLP toxicity/safety
under conditions that closely mimic human physiology.
These models allow longitudinal studies while significantly reducing the number of animals included in studies, thanks to non-invasive imaging technologies, when applicable.
Take advantage of robust in vitro systems to explore the mechanisms of action of drug candidates and generate reliable preliminary data.
These models enable fast and ethical screening before moving to in vivo animal experimentation.
Ex vivo models (organ cultures, tissues explants) preserve the tissue architecture and microenvironment,
allowing predictive and accurate analysis of treatment responses while adhering to the 3Rs principles.
Imavita offers image analysis services dedicated to preclinical studies.
The goal is to extract quantitative data (tumor volumes, tissue density, signal intensity, etc…) and qualitative insights (treatment effect visualization, disease progression tracking).
The analysis integrates multiple imaging modalities (BLI, FRI, OCT, PET/CT, etc.) using adapted tools and custom reporting.
Imavita offers tailored R&D services for the development of preclinical models, imaging protocols, and specific analytical methods.
We support exploratory and collaborative projects from proof of concept to the optimization of translational tools.
Each study is designed in close alignment with defined scientific goals in various domains of drug R&D.
Imavita provides pharmacokinetic (PK) studies adapted to preclinical models to characterize the absorption, distribution, metabolism, and elimination of drugs.
Our approach combines non-invasive imaging, quantitative analysis, and tailored protocols to assess biodistribution or time-course drug exposure.
- Exploratory or targeted studies supporting early-stage development
- Optional integration with PD data and imaging biomarkers
Discover our customized in vivo models designed to assess efficacy, pharmacokinetics (PK), and non-GLP toxicity under conditions that closely mimic human physiology. These models allow for longitudinal studies while significantly reducing the number of animals used, thanks to non-invasive imaging technologies, when applicable.
Take advantage of robust in vitro systems to explore the mechanisms of action of drug candidates and generate reliable preliminary data. These models enable fast and ethical screening before moving to animal experimentation.
Our ex vivo models (organ cultures,, tissues explants) preserve the tissue architecture and microenvironment, allowing predictive and accurate analysis of treatment responses while adhering to the 3Rs principles.
Imavita offers image analysis services dedicated to preclinical studies. The goal is to extract quantitative data (tumor volumes, tissue density, signal intensity, etc.) and qualitative insights (treatment effect visualization, disease progression tracking).
The analysis integrates multiple imaging modalities (BLI, FRI, OCT, PET/CT, etc.) using adapted tools and custom reporting.
Imavita offers tailored R&D services for the development of preclinical models, imaging protocols, and specific analytical methods. We support exploratory and collaborative projects from proof of concept to the optimization of translational tools. Each study is designed in close alignment with your scientific goals.
Imavita provides pharmacokinetic (PK) studies adapted to preclinical models to characterize the absorption, distribution, metabolism, and elimination of compounds.
Our approach combines non-invasive imaging, quantitative analysis, and tailored protocols to assess biodistribution or time-course drug exposure.
- Exploratory or targeted studies supporting early-stage development
- Optional integration with PD data and imaging biomarkers
Precise evaluation by :
- Classical approach using unlabeled compounds
- In vivo imaging approach after labeling.
State of the art models of:
- Psoriasis
- Atopic dermatitis
- Acne
- Skin wound healing
- Alopecia
- Pemphigus vulgaris
Why choose Imavita as your preclinical CRO partner for research?
Benefit from personalized guidance and tailored solutions for your pathology testing model
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Count on reliable and reproducible data
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Rely on advanced expertise for innovative solutions
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Accelerate your R&D
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Take advantage of Research Tax Credit Accreditation
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Be responsible
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Driving innovation in drug development with tailored preclinical services and cutting-edge imaging technologies.
«The team was very implicated in a challenging development of a specific dermatology model in skin healing.
Animals welfare was more than respected, and results were quickly released for dosage optimization modelling.»
S.D., Project leader PKPD
Europe-based Pharma company
Empowering biotech firms with advanced models and responsive solutions to accelerate R&D pipelines.
«Impressive turnover results with beautiful images permitted us to visually convince our investors in continuing the program on our chimeric biological candidate.
Quantification was a must for scientific advise.»
R.L., CSO
US-based Biotech company
Supporting academic and industrial research with specialized expertise and adaptable methodologies.
«The academic lab discovered an interesting small molecule for which pilot data were necessary to validate cancer efficacy.
Imavita was very efficient in implementing fast in vitro model for our specific needs.
In vivo data qualified our candidate for future tests.»
B.R., TTO Team Leader
France
Join leading pharmaceutical, biotech companies and R&D teams who trust Imavita for their
preclinical research needs.
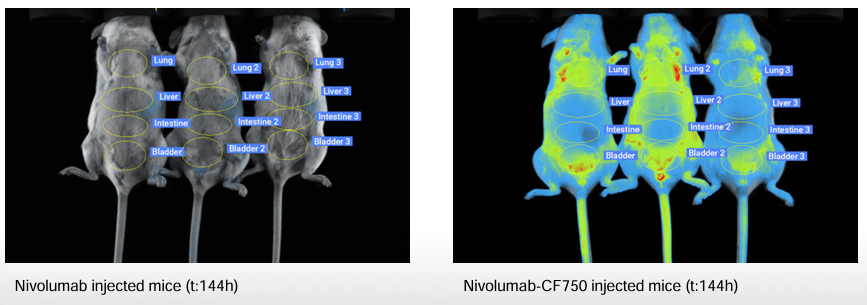
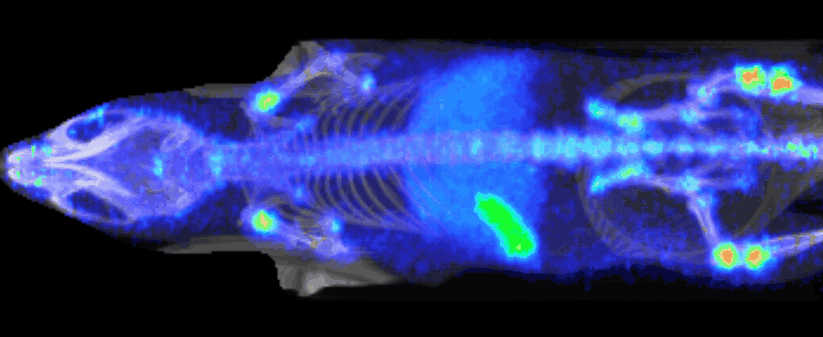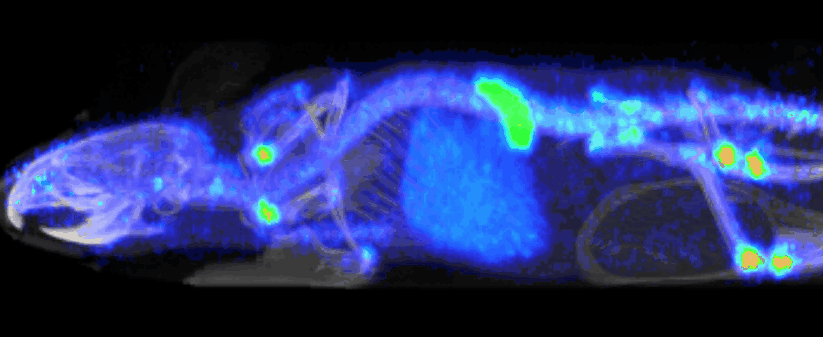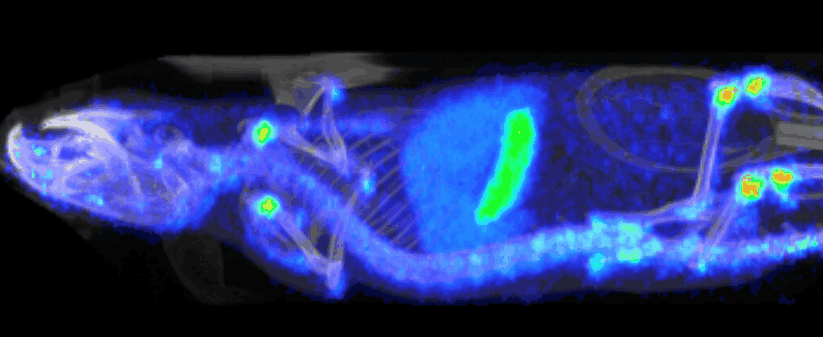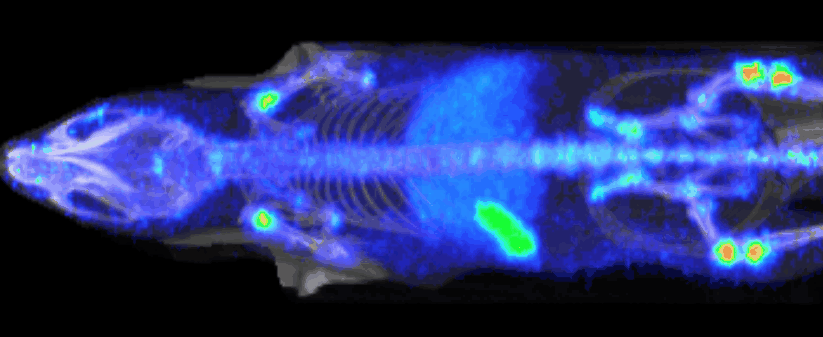
Image-Enhanced PK: IMAVITA’s Key Learnings in Preclinical Models
Imavita combines classical bioanalysis with near-infrared imaging to deliver organ-resolved, longitudinal PK and biodistribution data. Our end-to-end studies (sampling, in vivo/ex vivo imaging, and ELISA bridging) make PK
Image-Enhanced PK: IMAVITA’s Key Learnings in Preclinical Models
Imavita combines classical bioanalysis with near-infrared imaging to deliver organ-resolved, longitudinal PK and biodistribution data. Our end-to-end studies (sampling, in vivo/ex vivo imaging, and ELISA bridging) make PK
- News
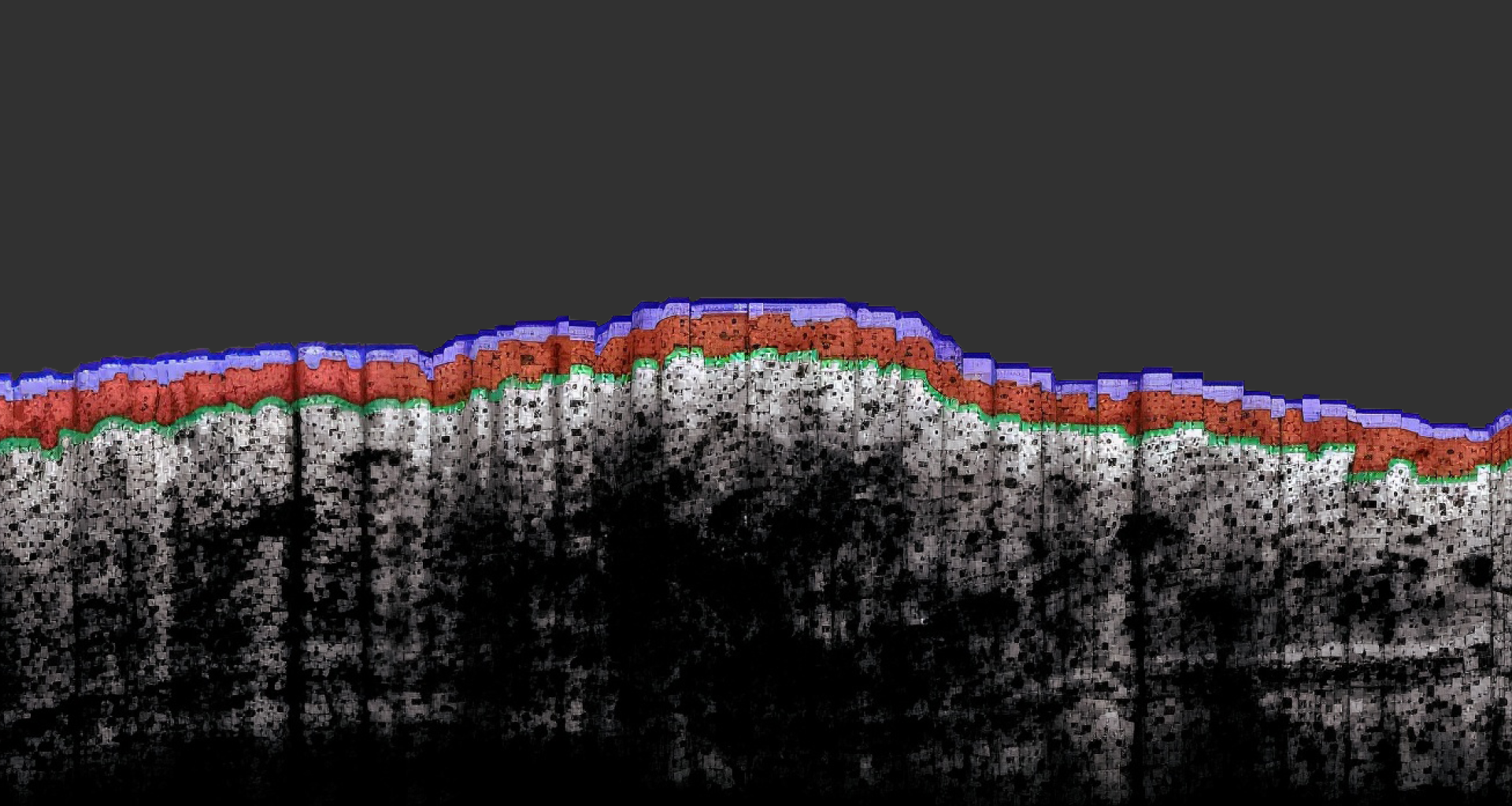
In vivo monitoring of chronic skin inflammation using OCT in preclinical dermatology models Chronic inflammatory skin diseases, such as atopic dermatitis, psoriasis, acne, or pemphigus vulgaris, significantly impact
In vivo monitoring of chronic skin inflammation using OCT in preclinical dermatology models Chronic inflammatory skin diseases, such as atopic dermatitis, psoriasis, acne, or pemphigus vulgaris, significantly impact
- News
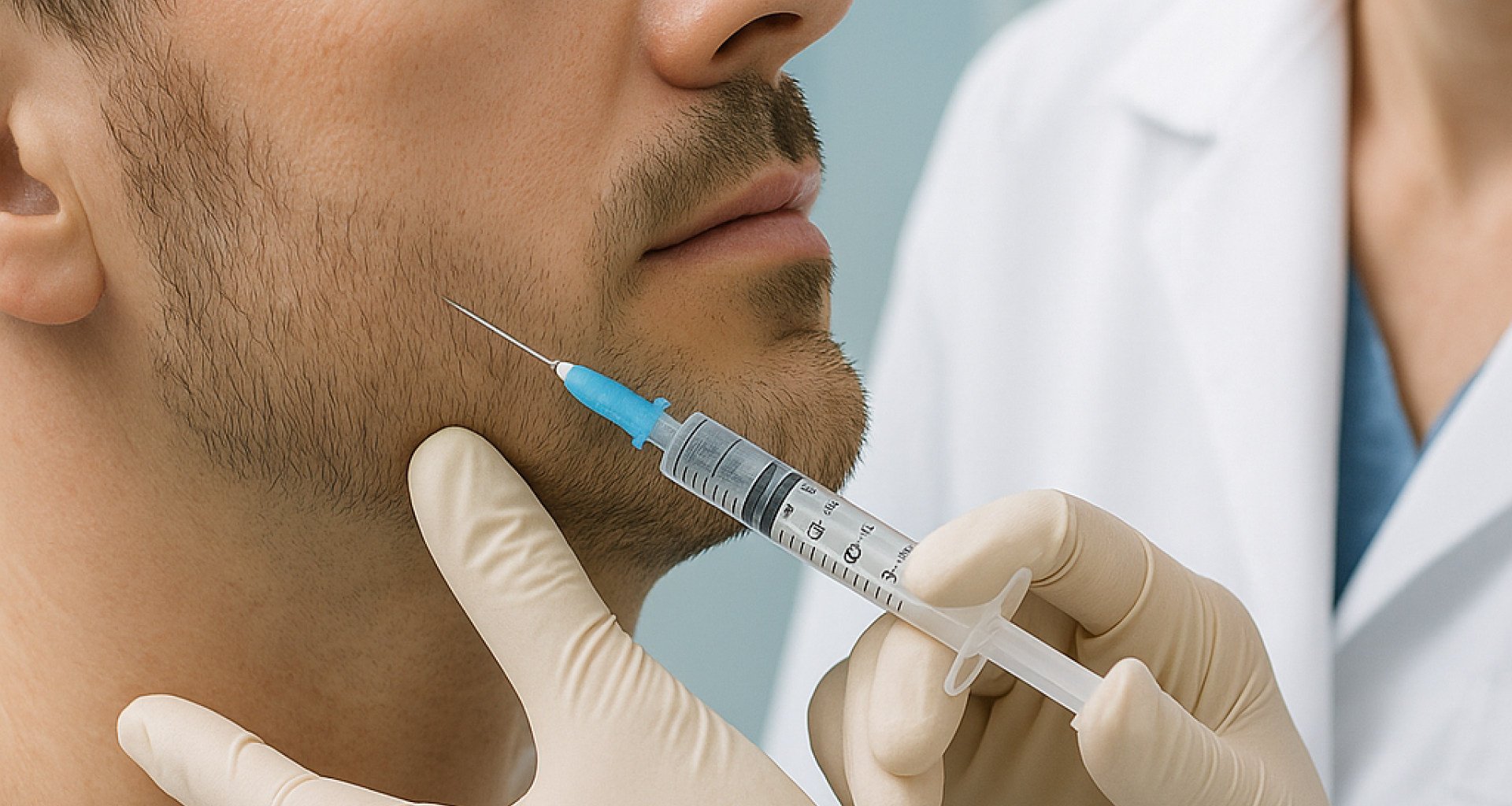
Botulinum toxin as a skin enhancer: Insights from preclinical dermatology models
This joint study by Imavita and the biopharmaceutical group Ipsen, published in late 2023 in Toxicon, compares the effects of a single injection of aboBoNT-A (botulinum toxin)
Botulinum toxin as a skin enhancer: Insights from preclinical dermatology models
This joint study by Imavita and the biopharmaceutical group Ipsen, published in late 2023 in Toxicon, compares the effects of a single injection of aboBoNT-A (botulinum toxin)
- News