Alopecia aerata / androgenic Mouse Model
Introduction / Alopecia aerata / androgenic Mouse Model
Alopecia preclinical models are necessary in R&D.
The medical term for the loss of hair on the head or body is alopecia. Alopecia can take many different forms, but the most prevalent ones are alopecia areata and androgenic alopecia. Alopecia is not a fatal condition, but it can have a significant psychological impact on those who have it and change their lives.
Hair loss from small, patchy bald spots to total loss of scalp or body hair is a defining feature of alopecia areata, a complex inflammatory illness. Although it can impact people of any age or gender, it typically shows up in childhood or early adulthood. The most popular treatments for alopecia include topical medications, which are intended to promote hair growth and control the immune system. Relapse is frequent, though, and the effectiveness of various treatments varies greatly.
Alopecia affects about 2% of people worldwide, making it a serious global health concern due to its unpredictable course and profound psychological effects.
In androgenetic (or pattern) alopecia, terminal hairs gradually turn into indeterminate and then vellus hairs, a genetically determined condition. The illness is quite widespread and can afflict both men and women.
Alopecia preclinical models were developed as models of alopecia androgenetic or areata at Imavita for R&D purpose.
Protocol summary / Alopecia aerata / androgenic Mouse Model
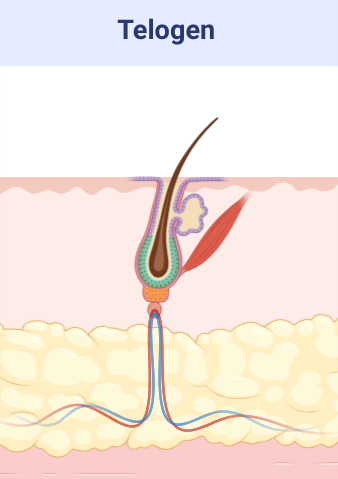
- Hair clipping at telogen phase.
- Mice details: Immunocompetent strain in SPF facility A1.
- Treatments administration: test drugs via topical, oral, IP or SC routes.
- Monitoring for maximum 40 days: bodyweights, hair growth scoring, visual macroscopic scoring (erythema, scaling), digital pictures.
- Ethical endpoints: bodyweight modifications / feeding & drinking behavior / general behavior.
- Evaluation of hair cycle: histology (HES / IF / IHC), genes expression (qPCR).
Typical results / Alopecia aerata / androgenic Mouse Model
- Visual hair regrowth
- Hair regrowth increase with minoxidil topical
- Confirmation by histology (proliferation / hair follicle stage)
Alopecia aerata / androgenic Mouse Model / Detailed Protocol
Alopecia Induction:
Alopecia induction by hair clipping of mice in telogen phase of hair growth
Animal strain:
– Normal mice strain at induction (immuno-competent)
– Strain C57Bl6 (inbred)
– Health status: SPF or SOPF facility after acclimatization about 1 week
– Immuno-competent at induction: age about 7 weeks
– Gender: Male or Female

Application of dermatological pharmaceutical products:
– Ready to use formulations / Formulation screening on request
– Topical or Oral (PO) or IP or SC routes
– Treatment duration: 4 to 6 weeks
– Alopecia inhibitors for reference or comparison (minoxidil / ciclosporin / JAKi).
Evaluation of in vivo clinical macroscopic signs:
– Body weight / General behavior
– Ear skin macroscopic description (twice per week):
- Hair regrowth scoring
- Erythema (skin redness) / Visual scoring or SkinColorCatch
- Scaling (skin dryness) / Visual scoring
- Other macroscopic skin observations

In vivo imaging:
– Digital pictures of ears
– Optional: Sub-macroscopical exploration using Optical Coherence Tomography (OCT)
Ex vivo evaluations:
- Histology HES (Hemalun-Eosin-Safran) after paraffin inclusion with the following histomorphometrics analysis:
- Epidermis thickness measurement
- Dermis inflammation (cellularity)
- Hair cycle
- Other noticeable observations
- Genes expression by qPCR on tissue extracts
- Cytokines assay using multiplexed ELISA
- Other ex vivo imaging techniques of interest:
- Specific stainings and imaging IHC or IF on request
- Specific imaging MALDI/MS on request (in partnership)

Alopecia aerata / androgenic Mouse Model / Conclusion
Preclinical animal models of alopecia are used to study the underlying mechanisms of the disease and to test potential treatments before they are evaluated in clinical trials involving humans.
8 to 10 animals per group are generally sufficient to underline anti-alopecia effect of new therapeutics (based on difference about 25 to 30%).
Various drugs can be used as alopecia inhibitors for reference or comparison (ciclosporin / minoxidil / JAKi).
By using this model, researchers can gain a better understanding and investigate potential anti-alopecia therapies.
However, it’s important to note that pre-clinical mouse models are only approximations of the human condition and may not completely reflect the human specificity.
Cautions to be taken on this model:
Does PK of the test drug need to be evaluated first?
Drug pharmacokinetics / ADME / transcutaneous passage should be known to optimize drug dosing whatever the route. This is recommended, but not required.
What excipient should be used for topical route?
Use of previously untested excipients should be avoided as they could cause false positive, false negative or local irritation. In any case, excipient negative group will be included.
Which requirements are needed for formulation?
Fomulation physico-chemistry should be well known (pH, osmolarity, etc…) as these factors can impact efficacy testing, principally for topical applications.
Project duration and follow-up:
Approximated duration 60 days for in vivo experiment until draft report for in vivo.
- Protocol setup and approval / 1-2 weeks
- In vivo experimentation / 5 weeks (Can be shortened if 8 days induction scheme is applied)
- In vivo results / 1 week
- Draft report 1st version / 2 weeks
- Additional optional qPCR genes expression analysis / 1-2 weeks
- Additional optional proteins analysis using multiplexed ELISA / 1-2 weeks
- Additional optional histology / 6-7 weeks
Do not hesitate to contact us if you need more information or a quotation on this model.
Alopecia aerata / androgenic Mouse Model / Bibliography
- Flores, A., Choi, S., Hsu, Y. & Lowry, W. E. Inhibition of pyruvate oxidation as a versatile stimulator of the hair cycle in models of alopecia. Exp. Dermatol. 30, 448–456 (2021).
- Liu, X. et al. Development of Novel Mitochondrial Pyruvate Carrier Inhibitors to Treat Hair Loss. J. Med. Chem. 64, 2046–2063 (2021).
- Wang, E. et al. Development of Autoimmune Hair Loss Disease Alopecia Areata Is Associated with Cardiac Dysfunction in C3H/HeJ Mice. PLoS One 8, e62935 (2013).
- Gilhar, A. et al. Autoimmune Disease Induction in a Healthy Human Organ: A Humanized Mouse Model of Alopecia Areata. J. Invest. Dermatol. 133, 844–847 (2013).
- Wikramanayake, T. C. et al. Heat treatment increases the incidence of alopecia areata in the C3H/HeJ mouse model. Cell Stress Chaperones 15, 985–991 (2010).
- Nakamura, M., Jo, J., Tabata, Y. & Ishikawa, O. Controlled Delivery of T-box21 Small Interfering RNA Ameliorates Autoimmune Alopecia (Alopecia Areata) in a C3H/HeJ Mouse Model. Am. J. Pathol. 172, 650–658 (2008).
- McElwee, K. J., Freyschmidt-Paul, P., Zöller, M. & Hoffmann, R. Alopecia areata susceptibility in rodent models. J. Investig. Dermatology Symp. Proc. 8, 182–187 (2003).
- Porter, R. M. Mouse models for human hair loss disorders. J. Anat. 202, 125–31 (2003).
- Müller-Röver, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3–15 (2001).
- Militzer, K. & Wecker, E. Behaviour-associated alopecia areata in mice. Lab. Anim. 20, 9–13 (1986).

