Preclinical services for the pharmaceutical, biotechnological, academic and private sectors
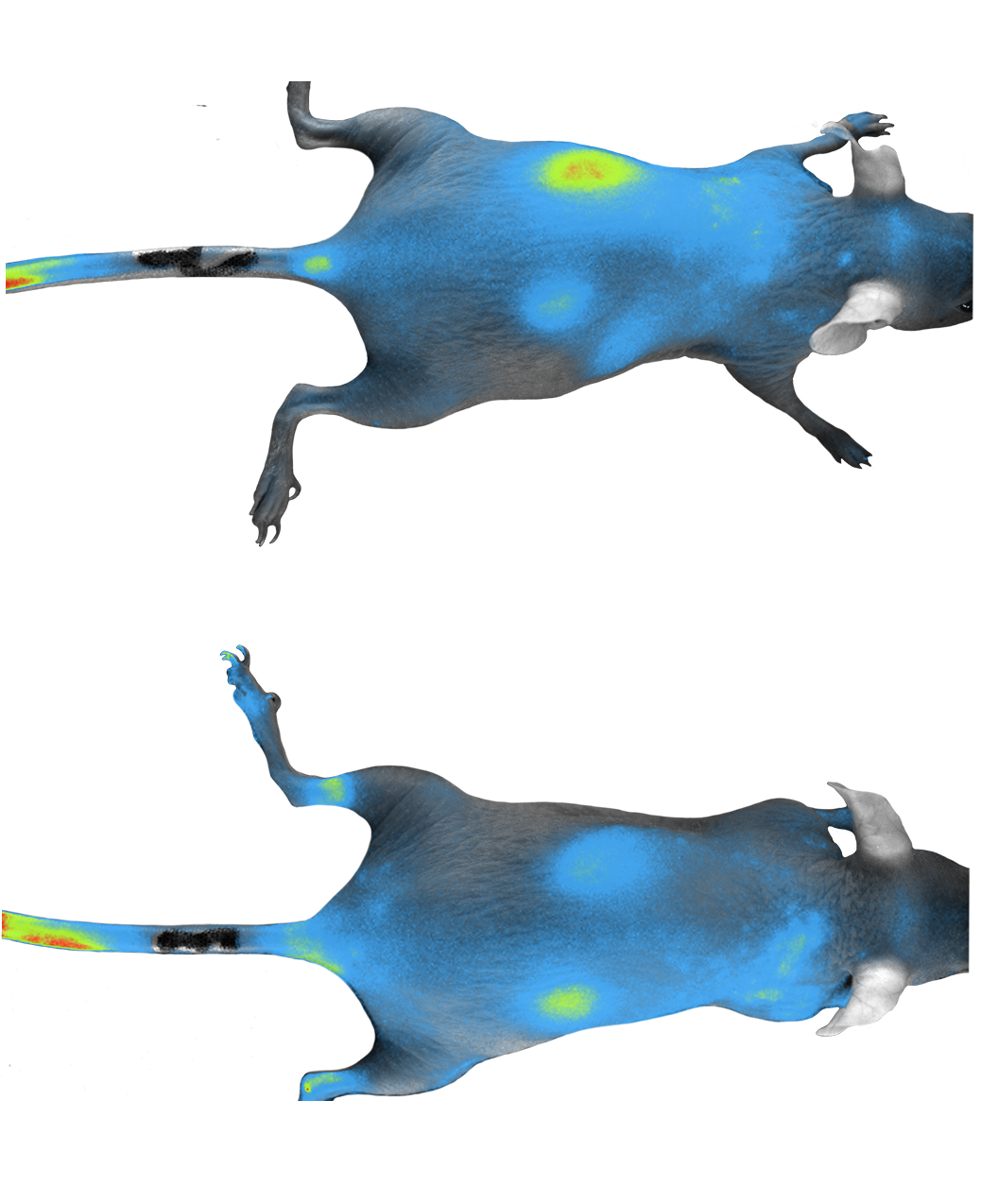
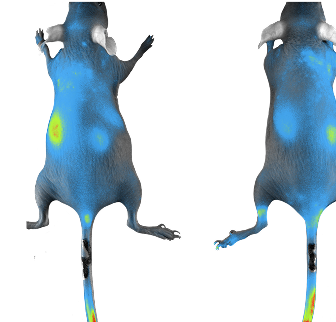
Through preclinical testing, including pharmacodynamics, pharmacokinetics and toxicology evaluations in in vitro and in vivo settings, is essential to identify safe, potent, and effective drugs.
However, only a small fraction of investigational drugs successfully transition from clinical trials to marketed products, as developing a new drug is time consuming
and costly.
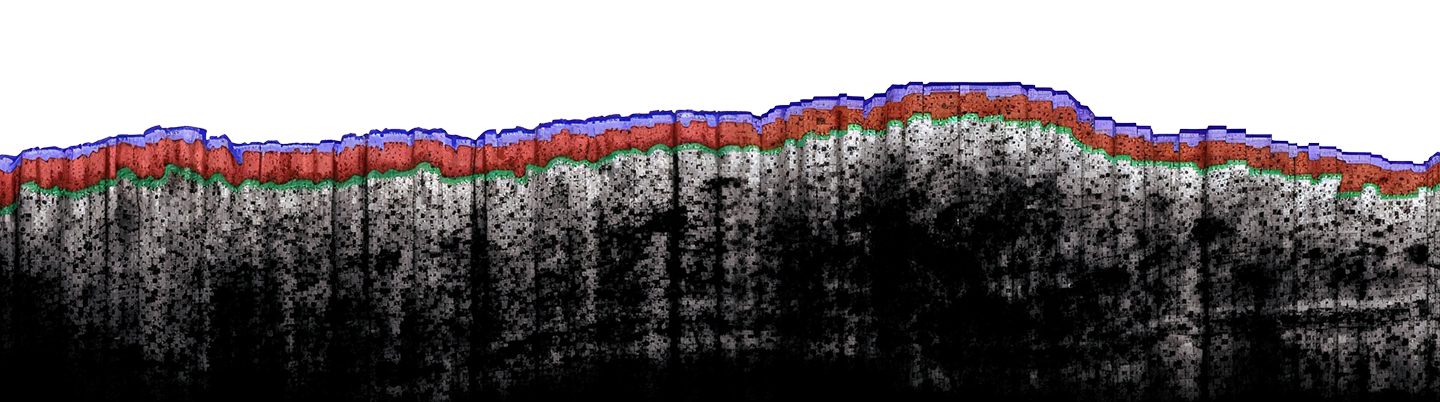
Comprehensive evaluations to drive preclinical development of drugs and new molecules
In preclinical research, PK evaluation is essential for optimizing dosing strategies, predicting therapeutic benefits, and minimizing adverse effects.
Benefit from personalized guidance and tailored solutions for your pathology testing model
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Count on reliable and reproducible data
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Rely on advanced expertise for innovative solutions
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Accelerate your R&D
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Take advantage of Research Tax Credit Accreditation
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Be responsible
We provide tailor-made support to design effective studies and meet specific needs, offering adaptability larger organizations cannot match
Dedicated support to design effective studies and address your specific needs with precision.
Contact Imavita and discover how we can support your studies toward developing innovative therapies.

