How IMAVITA applies the 3Rs in preclinical studies thanks to its models and the use of imaging

Applying the 3Rs in preclinical studies: IMAVITA’s vision and practices
Before being approved for use, drugs, chemicals and new therapeutics must demonstrate their efficacy and safety through preclinical studies. Animal experimentation has long been a central pillar of this research. While it has led to major scientific breakthroughs, it continues to raise ethical concerns that have been debated for decades. For more than 60 years, the scientific community has worked toward adopting more ethical and responsible practices, guided by the 3Rs principle. Today, these values are enshrined in regulatory frameworks and integrated into the strategies of many organizations — including IMAVITA, a CRO specialized in preclinical models and imaging. This article reviews the regulatory foundations and applications of the 3Rs in preclinical studies.
Regulatory framework for animal experimentation in preclinical research
EU regulations on the use of animals for scientific purposes
Historically, the Nuremberg Code was established in 1947 in response to the atrocities committed by medical researchers during World War II. It set out the first internationally recognized ethical principles for human experimentation, emphasizing voluntary consent and the protection of human subjects. Animal experimentation was required by the Code (3rd point of the 10 points of Nuremberg Code): “3. The experiment (to be performed in human subjects, Note from author) should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results will justify the performance of the experiment.” The Nuremberg Code was completed in June 1964 by the World Medical Association (WMA) Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects adopted by the 18th WMA General Assembly, Helsinki, Finland (Point 21 of Declaration of Helsinki): “21. Medical research involving human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and adequate laboratory and, as appropriate, animal experimentation. The welfare of animals used for research must be respected.” Within the European Union, the 3Rs principle – Replacement, Reduction, and Refinement – is a core ethical guideline governing the use of animals in scientific experimentation. First introduced by Russell and Burch in 1959, it is now enshrined in Directive – 2010/63 – EN – EUR-Lex, which regulates the protection of animals used for scientific purposes across all member states. This directive mandates the replacement of animals whenever a reliable alternative method is available, the reduction of animal use to the minimum necessary, and the refinement of procedures to minimize pain, suffering, and distress. Implementation of these principles is overseen by ethics committees and national competent authorities in each country, which evaluate and approve research projects prior to their launch. The goal is to ensure a high level of animal welfare while maintaining the scientific quality of research.Regulatory focus on the application of the 3Rs by the FDA in the United States
In the United States, the FDA, through its Center for Devices and Radiological Health (CDRH), has recently published several documents encouraging the use of replacement, reduction, and refinement strategies in in vivo testing.
As part of the Food and Drug Administration Safety and Innovation Act, and particularly through fast-track programs such as the Breakthrough Therapy Designation, the FDA promotes:
- in certain cases, the use of large animals, which allows for better clinical translatability and reduces the need for repeated studies — thereby decreasing overall animal use;
- the implementation of integrated experimental designs, combining clinical data, imaging, diagnostics, and pathological analyses;
- the application of scientific principles, methods, and endpoints (SPME) to ensure the validity and robustness of the results.
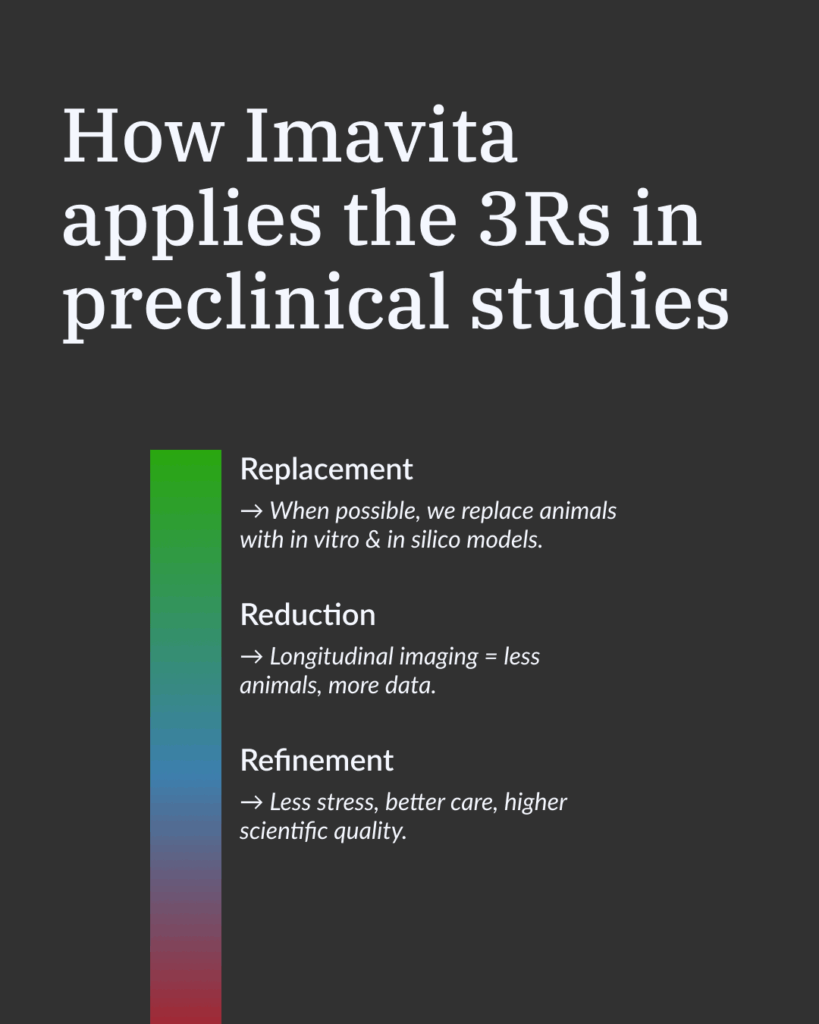
The 3Rs in preclinical studies at IMAVITA
The 3Rs principle aims to uphold animal welfare without compromising the robustness of scientific results. At IMAVITA, ethical approaches to animal experimentation are implemented through several key practices:
- Our medium-sized animal models are designed to meet SPME requirements.
- Our non-invasive imaging tools (Fluorescence FLI/ FRI, Bioluminescence BLI, Optical Coherence Tomography OCT, Ultrasonography, Laser Doppler LDI, PET/CT, MRI, etc…) enable longitudinal monitoring without the need for euthanasia.
- Our integrated experimental designs maximize the data collected from each individual, thereby reducing the number of animals required.
This alignment between scientific rigor, regulatory compliance, and ethical commitment confirms the relevance of our 3Rs-centered approach.
The European Union has integrated the 3Rs principles into its legal framework for animal experimentation. Researchers are obligated to respect these principles, ensuring that animal experiments are conducted only when necessary and with the welfare of the animals as a primary consideration.
The Declaration of Helsinki and the 3Rs principles continue to guide medical research involving human subjects and animal experimentation, respectively. The WMA and other international organizations emphasize the importance of ethical considerations in all aspects of medical research.
What is the 3Rs principle: Replacement, Reduction, Refinement?
1. Replacement: alternatives to replace animals in preclinical research
Replacement aims to avoid the use of animals whenever possible. Various methods can contribute to this goal:
- Use of in vitro research on cells or tissues: 2D or 3D cell cultures, organoids, or organ-on-chip systems are increasingly capable of reproducing human biological functions with high fidelity.
- Use of in silico models: QSAR models, ADMET simulations, molecular docking, and now Artificial Intelligence all help predict the toxicity or efficacy of a compound without relying on animal testing.
However, these alternative methods are complementary to the use of animal models. To date, they cannot fully replace them due to several limitations:
- Disease modeling remains imperfect
- Predicting side effects of monoclonal, gene, or cell therapies is still unreliable
- Understanding of biological mechanisms is limited, and available data are often insufficient
A thorough bibliographic approach in research should also not be overlooked, as in-depth analysis of existing literature can prevent unnecessary experiments and help answer certain scientific hypotheses.
2. Reduction: minimizing the number of animals used
When animal experimentation is necessary, it is essential to optimize protocols to use the minimum number of animals:
- Smart experimental design: rigorous methodologies, multicenter studies, and well-planned, robust statistical analyses
- Data reuse and sharing
- Robust statistical models that allow for smaller sample sizes while maintaining result validity
- Validation through complementary methods: combining in vitro, ex vivo, and in silico approaches to reduce the need for animal studies
- Use of imaging tools (Fluorescence FLI/ FRI, Bioluminescence BLI, Optical Coherence Tomography OCT, Ultrasonography, Laser Doppler LDI, PET/CT, MRI, etc…) that enable monitoring treatment progression in animals, reducing the need for invasive sampling and euthanasia
In research, the publication of negative results and data transparency (following the FAIR principles of Findability, Accessibility, Interoperability, and reusability) help avoid unnecessary duplication and promote more ethical animal use.
The importance given to reproducibility also aims to maximize the knowledge obtained for each animal used, which is in line with the idea of reduction.
3. Refinement: improving practices to reduce animal suffering
Refinement aims to prioritize animal welfare by minimizing pain and distress. This can be achieved through:- the use of less invasive protocols (i.e. non-invasive imaging)
- environmental enrichment, socialization and appropriate care
- monitoring of pain and the administration of analgesics
- ongoing training of researchers in best practices
- the use of in vivo imaging, which helps avoid invasive sampling and the euthanasia of animals
In vivo imaging: an effective tool to reduce animal use
As part of the application of the 3Rs in preclinical studies, imaging is a powerful tool for reducing the number of animals used while increasing both the quantity and quality of the data collected.
Thanks to techniques such as Fluorescence FLI/ FRI, Bioluminescence BLI, Optical Coherence Tomography OCT, Ultrasonography, Laser Doppler LDI, PET/CT, MRI, etc…, it is now possible to:
- Monitor the same animal over time, without euthanasia or destructive sampling
- Track the progression of a disease or the response to treatment in a single individual, which improves statistical power and can reduce the number of animals used by a factor of 2 to 5 in certain studies
- Use each animal as its own control, thereby reducing inter-individual variability
This longitudinal follow-up avoids the need for intermediate euthanasia, which would otherwise be required for fixed time-point analyses. It also improves the robustness of results by allowing researchers to observe biological dynamics within the same subject.
At IMAVITA, integrating imaging into study protocols is central to our 3Rs approach. Imaging provides valuable physiopathological information in a non-invasive way, helping to refine procedures while reducing the number of animals used.
In dermatology: human skin models as alternatives to animal use
In certain conditions such as atopic dermatitis, psoriasis, acne or rosacea, the limitations of animal models are increasingly recognized. Despite advances in understanding this chronic inflammatory skin disease, interspecies differences often hinder the translation of results to humans.
To address these limitations, ex vivo skin models have emerged as credible alternatives to animal experimentation, particularly for:
- Exploring the pathophysiological mechanisms of atopic dermatitis
- Assessing skin toxicity or immune responses to new formulations
- Testing therapeutic candidates under conditions that closely mimic human skin
At IMAVITA, we incorporate ex vivo models of human skin into our preclinical dermatology studies. This allows us to assess key parameters—such as inflammation, permeability, and regeneration—without using live animals.
In our preclinical models of atopic dermatitis, psoriasis, rosacea and acne, we have used Optical Coherence Tomography (OCT), a non-invasive imaging technique particularly suited to chronic skin conditions. This approach demonstrates the application of the 3Rs principles on several levels:
- Reduction of animal use: by enabling longitudinal monitoring of lesions in the same individual, OCT reduces the need for new subjects at each time point
- Refinement of protocols: imaging is performed in vivo, without contact or pain, avoiding destructive sampling and intermediate euthanasia
- Time and efficiency gains: whereas histological analysis may take several weeks, an OCT scan takes only a few minutes and provides real-time, actionable data
- Validated correlation with histology: epidermal thickness measurements obtained via OCT show excellent agreement with histological sections (r² = 0.84), ensuring scientific robustness
- Expansion to other models: IMAVITA also applies OCT in models of wound healing, ophthalmology, and acne, reinforcing its value in an ethical and versatile preclinical approach
In vitro models for ethical and effective science: a case study in immunomodulation and viral oncology
In the context of human papillomavirus (HPV), a key driver in cervical carcinogenesis, a recent study (Jacques et al., 2024), to which IMAVITA contributed, highlights the relevance of human in vitro models in the application of the 3Rs in preclinical studies.
Two cellular models were used, without any reliance on animal testing:
- Human primary immune cells (PBMCs) exposed to peptides derived from HPV-16, simulating a persistent infection context
- HeLa cell line (cervical cancer, HPV-18 positive), cultured under stress conditions (serum deprivation)
Using these systems, the study demonstrated:
- An immunomodulatory effect of the candidate compound, notably through modulation of key cytokines (IL-6, IFN-γ, IP-10)
- Targeted lymphocyte activation, suggesting enhanced cellular immune responses
- An antiproliferative effect on tumor cells, mimicking a therapeutic response
This approach demonstrates the power of in vitro models to simulate complex tumor–immune system interactions, while adhering to an ethical framework that avoids animal experimentation.
In conclusion, applying the 3Rs in preclinical studies is no longer a mere recommendation — it is a scientific, regulatory, and ethical imperative. By combining technological innovation, experimental expertise, and alternative models, IMAVITA as a CRO shows that it is possible to deliver high-level research while minimizing animal use in experiments.
Developing a preclinical research program ?
Contact our scientific team to discover how IMAVITA puts technology and ethics at the service of your goals.
References
Hampshire VA, Gilbert SH. Refinement, Reduction, and Replacement (3R) Strategies in Preclinical Testing of Medical Devices. Toxicol Pathol. 2019 Apr;47(3):329-338. doi: 10.1177/0192623318797289.
Obrecht M, Zurbruegg S, Accart N, Lambert C, Doelemeyer A, Ledermann B, Beckmann N. Magnetic resonance imaging and ultrasound elastography in the context of preclinical pharmacological research: significance for the 3R principles. Front Pharmacol. 2023 Jun 28;14:1177421. doi: 10.3389/fphar.2023.1177421.
Löwa A, Jevtić M, Gorreja F, Hedtrich S. Alternatives to animal testing in basic and preclinical research of atopic dermatitis. Exp Dermatol. 2018 May;27(5):476-483. doi: 10.1111/exd.13498.
Spanagel R. Ten Points to Improve Reproducibility and Translation of Animal Research. Front Behav Neurosci. 2022 Apr 21;16:869511. doi: 10.3389/fnbeh.2022.869511.
Jacques C, Marchand F, Chatelais M, Albinet V, Coustal C, Floris I. The Micro-Immunotherapy Medicine 2LPAPI® Displays Immune-Modulatory Effects in a Model of Human Papillomavirus Type-16 L1-Protein Capsid-Treated Human Peripheral Blood Mononuclear Cells and Antiproliferative Effects in a Model of Cervical Cancer Cells. Cancers (Basel). 2024 Apr 5;16(7):1421. doi: 10.3390/cancers16071421.
