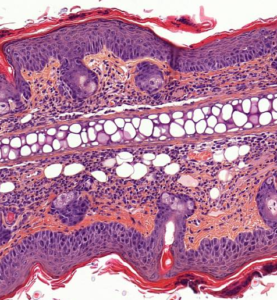
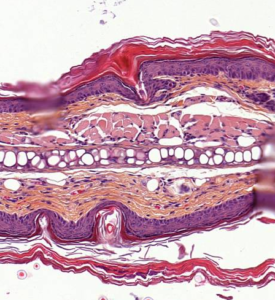
Imiquimod-Induced Psoriasis Model
Overview of the IMQ-induced Psoriasis Mouse Model
Introduction
Imiquimod (IMQ) produces a cutaneous phenotype in mice frequently studied as an acute model of human psoriasis.
IMQ is a Toll-like Receptor (TLR7) agonist that can be applied to mouse skin to elicit erythema, scaling, epidermis hyperplasia, hyperkeratosis, parakeratosis and dermis inflammation. IMQ induces also IL-17/ IL-23 axis cytokines.
Imiquimod-Induced Psoriasis Mouse Model is a convenient, easy-to-use and affordable mouse model of acute inflammatory response, which is widely used in mechanistic pharmacology of pathology and as a pre-clinical animal model for drug screening and testing before clinical testing on volunteers psoriatic patients.
Protocol Summary
- Immunocompetent strains topically applied with IMQ for 5 days
- Evaluation of in vivo parameters:
- Bodyweights / Ear- back tickness
- Macroscopic scoring (erythema / scaling / etc…)
- Epidermis thickness using OCT
- Evaluation of ex vivo parameters:
- Cytokines analysis / Cytometry
- Histology / Anatomo-pathological evaluation

Typical Results
IMQ-induced Psoriasis Mouse Model / Experimental Protocol
- Immunomodulator induction / imiquimod topical application
- Immunocompetent rodent sp. (i.e. Balb/c & C57Bl6)
- Environment status is important (SPF vs. non SPF conventional housing)
- Evaluation of:
- Skin thickness measurement (ear / back)
- Erythema / Scaling (Clinical endpoints) / Total scoring
- Blood samplings for circulating and skin biomarkers analysis (cytokines)
- In vivo imaging (OCT)
- Ex vivo imaging (histology HES / IHC : IF)
Typical Results and Pharmacological Validation / IMQ-induced Psoriasis Mouse Model
- Sham vs. IMQ-induction typical results:
- Increase of ear & back thickness
- Epidermis thickness / Dermis inflammation increase
- IMQ-induction vs. reference drug typical results:
- Reference drugs: corticoid (topical or oral) / immunosupressors (oral) / TNF inhibitors
- Reduction of ear & back thickness
- Epidermis thickness / Dermis inflammation decrease
Conclusion: Strengths and Use of the IMQ Psoriasis Model
Imiquimod-Induced Psoriasis Mouse Model is a first intention model, fast and reproducible used a routine model at Imavita.
8 subjects per group are generally sufficient to underline anti-psoriasis effect of new therapeutics (based on difference of at least 20 to 30% of ear / back thickness / epidermis thickness).
Cautions to be taken on this model:
- Housing conditions are important (SPF vs. conventional). Imavita perform this experiment in conventional conditions where results are more reproducible.
- Use of previously untested excipients should be avoided via topical route as they could cause false positive, false negative or local irritation.
- Formulation physico-chemistry must be well known (pH, osmolarity, etc…) as impact on results can be important.
- Drug pharmacokinetics / ADME / transcutaneous passage should be known to optimize dosing.
Bibliography & Scientific support for IMQ-induced Psoriasis Mouse Model
Do not hesitate to contact us if you need more information or a quotation on this model.