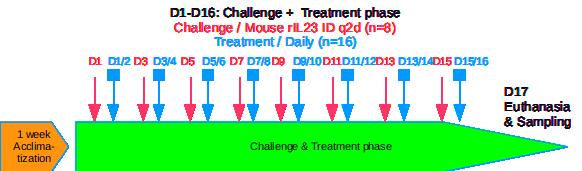
IL-23 induced Atopic Dermatitis Mouse Model
Introduction / IL-23 induced Atopic Dermatitis Mouse Model
IL-23 is produced by various immune cells, including dendritic cells and macrophages, and stimulates the activity of T helper 17 (Th17) cells. Th17 cells, in turn, produce cytokines that promote inflammation and recruit immune cells to the site of infection or injury. Studies have shown that levels of IL-23 are elevated in the skin lesions of patients with AD, and that blocking the activity of IL-23 can reduce the severity of the disease. Additionally, genetic studies have identified several genes that are associated with increased risk of AD, many of which are involved in the IL-23 signaling pathway.
Protocol summary / IL-23 induced Atopic Dermatitis Mouse Model
- Recombinant murine IL-23 intradermal injections.
- Mice details: Immunocompetent strain in SPF facility A1.
- Treatments administration: test drugs via oral, IP, IV, SC or topical routes.
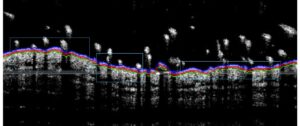
- Monitoring for 8 days to maximum 16 days: bodyweights, daily calipering (ears thickness), visual macroscopic scoring (erythema, scaling), SkinColor® (erythema), digital pictures, OCT (epidermis/dermis thickness), BLI, FLI.
- Ethical endpoints: bodyweight modifications / feeding & drinking behavior / general behavior / maximal AD scoring threshold
- Evaluation of inflammation: histology (HES), genes expression (qPCR) and cytokines levels (multiplexed ELISA) in ears.
Typical results / IL-23 induced Atopic Dermatitis Mouse Model
IL-23 induced Atopic Dermatitis Mouse Model / Detailed Protocol
IL-23 Induction:
Recombinant murine IL-23 / rIL-23 from commercial source: Produced in Spodoptera frugiperda, Sf 21 (stably transfected)-derived mouse IL-23 protein
IL-23 is a heterodimeric cytokine composed of two disulfide-linked subunits, a p19 subunit that is unique to IL-23, and a p40 subunit that is shared with IL-12. The p40 subunit is homologous to the extracellular domains of the hematopoietic cytokine receptors. The p19 subunit has homology to the p35 subunit of IL-12, as well as to other single chain cytokines such as IL-6 and IL-11. Mouse p19 cDNA encodes a 196 amino acid residue (aa) precursor protein with a putative 19 aa signal peptide and 177 aa mature protein. Human and mouse p19 share 70% sequence identity. Although p19 is expressed by activated macrophages, dendritic cells, T cells, and endothelial cells, only activated macrophages and dendritic cells express p40 concurrently to produce IL-23. The functional IL-23 receptor complex consists of two receptor subunits, the IL-12 receptor beta 1 subunit (IL-12 Rβ1) and the IL-23-specific receptor subunit (IL-23 R).
IL-23 has biological activities that are similar to, but distinct from IL-12. Both IL-12 and IL-23 induce proliferation and IFN-γ production by human T cells. While IL-12 acts on both naïve and memory human T cells, the effects of IL-23 is restricted to memory T cells. In mouse, IL-23 but not IL-12, has also been shown to induce memory T cells to secret IL-17, a potent proinflammatory cytokine. IL-12 and IL-23 can induce IL-12 production from mouse splenic DC of both the CD8- and CD8+ subtypes. However only IL-23 can act directly on CD8+ DC to mediate immunogenic presentation of poorly immunogenic tumor/self peptide.
Sequence: P43432 (p40) & Q9EQ14 (p19)
Quality control (by supplier):
– In vitro activity for IL-17 secretion on murine splenocytes (ED50 in 0.05-0.25 ng/mL)
– SDS-PAGE: >95% (Silver Staining and quantitative densitometry by Coomassie® Blue Staining) / MW 58-71 kDa
– Endotoxins (LAL method): <1.0 EU/μg
Intradermal injection q2d in ear(s) under flash gas anesthesia (isofluran)
Animal strain:
- Normal mice strain at induction (immuno-competent)
- Strain C57Bl6 (inbred)
- Health status: SPF or SOPF facility after acclimatization about 1 week
- Immuno-competent at induction: age > 7 weeks
- Gender: Male or Female

Application of dermatological pharmaceutical products:
- Ready to use formulations / Formulation screening on request
- Topical or Oral (PO) or IV or IP or SC routes
- Treatment duration: Minimum 8 days (short screening) / Up to 16 days (long treatment / dose response / benchmarking)
- Reference drugs: Anti-inflammatory drug (betamethasone topical or PO or IP)
Evaluation of in vivo clinical macroscopic signs:
- Body weight / General behavior
- Ear skin thickness (calipering every 2 days prior rIL-23 injection)
- Ear skin macroscopic description (every 2 days):
- Digital picture
- Erythema (skin redness) / Visual scoring or SkinColor
- Scaling (skin dryness) / Visual scoring
- Other macroscopic skin observations

In vivo imaging:
- Digital pictures of ears
- Bioluminescence BLI or Fluorescence FLI with specific probes
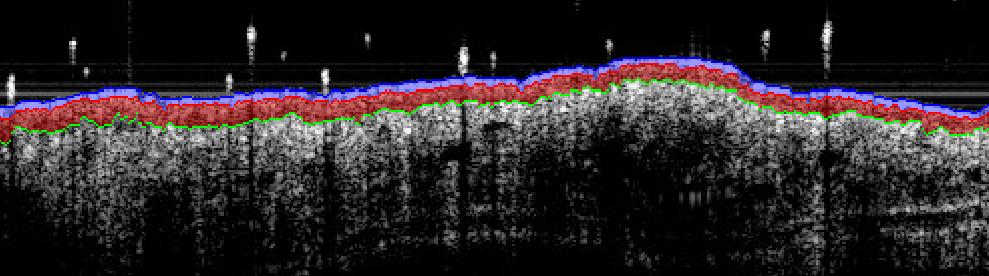
- Sub-macroscopical exploration using Optical Coherence Tomography (OCT):
- Epidermis thickness measurement
- Longitudinal at different times
- Prior ears collection & before fixation for histology
Ex vivo evaluations:
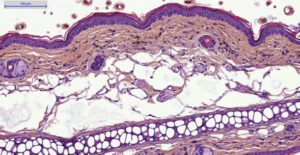
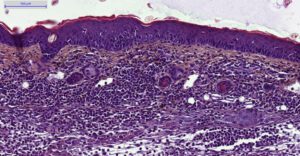
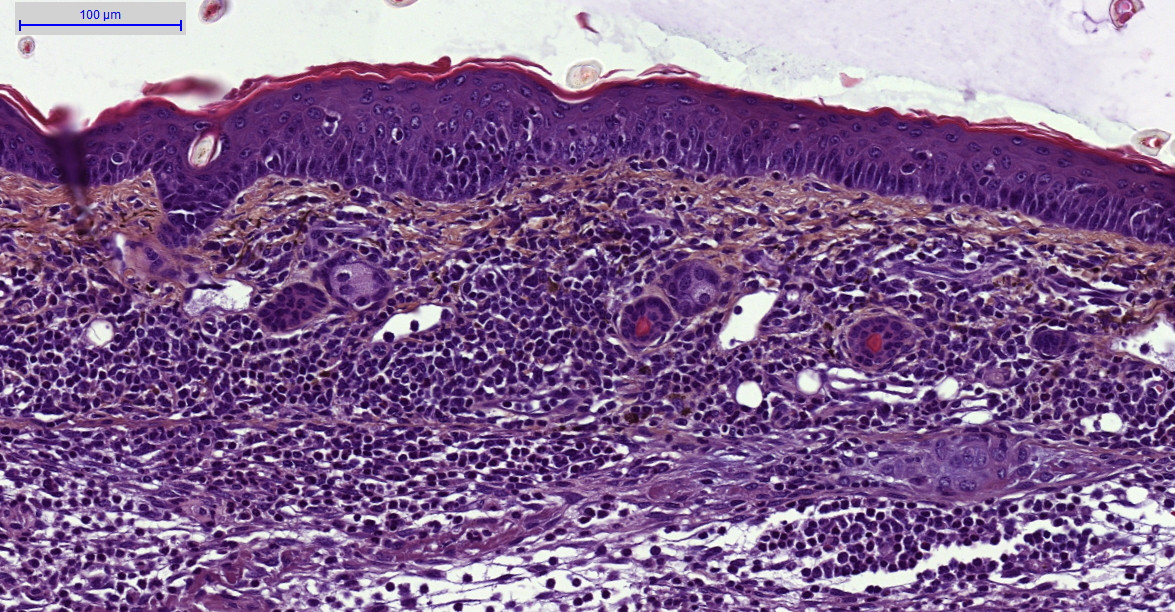
- Histology HES (Hemalun-Eosin-Safran) after paraffin inclusion with the following histomorphometrics analysis:
- Epidermis thickness measurement
- Dermis inflammation (cellularity)
- Dermis inflammation (tickness)
- Other noticeable observations (parakeratosis, neutrophilic abscess, …)
- Genes expression by qPCR on tissue extracts
- Cytokines assay using multiplexed ELISA
- Other ex vivo imaging techniques of interest:
- Specific stainings and imaging IHC or IF on request (in partnership)
- Specific imaging MALDI/MS on request (in partnership)
L-23 induced Atopic Dermatitis Mouse Model / Conclusion
Preclinical animal models of atopic dermatitis are used to study the underlying mechanisms of the disease and to test potential treatments before they are evaluated in clinical trials involving humans.
This IL-23 induced mouse model is a 1st intention model, fast and reproducible validated, with anti-inflammatory compounds as reference drugs.
8 to 10 animals per group are generally sufficient to underline anti-AD effect of new therapeutics (based on difference about 25 to 30%).
By using this model, researchers can gain a better understanding and investigate potential anti-AD therapies.
However, it’s important to note that pre-clinical mouse models are only approximations of the human condition and may not completely reflect the human specificity.
Cautions to be taken on this model:
Does PK of the test drug need to be evaluated first?
Drug pharmacokinetics / ADME / transcutaneous passage should be known to optimize drug dosing whatever the route. This is recommended, but not required.
Use of previously untested excipients should be avoided as they could cause false positive, false negative or local irritation. In any case, excipient negative group will be included.
Fomulation physico-chemistry should be well known (pH, osmolarity, etc…) as these factors can impact efficacy testing, principally for topical applications.
Project duration and follow-up:
Approximated duration 35 days for in vivo experiment until draft report for in vivo.
- Protocol setup and approval / 1-2 weeks
- In vivo experimentation / 3 weeks
Can be shortened if 8 days induction scheme is applied. - In vivo results / 1 week
- Draft report 1st version / 2 weeks
- Additional optional qPCR genes expression analysis / 1-2 weeks
- Additional optional proteins analysis using multiplexed ELISA / 1-2 weeks
- Additional optional histology / 6-7 weeks
Do not hesitate to contact us if you need more information or a quotation on this model.
IL-23 induced Atopic Dermatitis Mouse Model / Bibliography
1. Ewald, D. A. et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J. Allergy Clin. Immunol. 139, 562–571 (2017).
2. Bromley, S. K., Larson, R. P., Ziegler, S. F. & Luster, A. D. IL-23 Induces Atopic Dermatitis-Like Inflammation Instead of Psoriasis-Like Inflammation in CCR2-Deficient Mice. PLoS One 8, e58196 (2013).
3. Avci, P. et al. Animal models of skin disease for drug discovery. Expert Opin. Drug Discov. 8, 331–355 (2013).
4. Petersen, T. K. In vivo pharmacological disease models for psoriasis and atopic dermatitis in drug discovery. Basic Clin. Pharmacol. Toxicol. 99, 104–15 (2006).
5. Jin, H., He, R., Oyoshi, M. & Geha, R. S. Animal Models of Atopic Dermatitis. J. Invest. Dermatol. 129, 31–40 (2009).