Cutibacterium acnes C. acnes Acne Model / Overview
Acne vulgaris is a common inflammatory disorder that affects over 80% of adolescents.
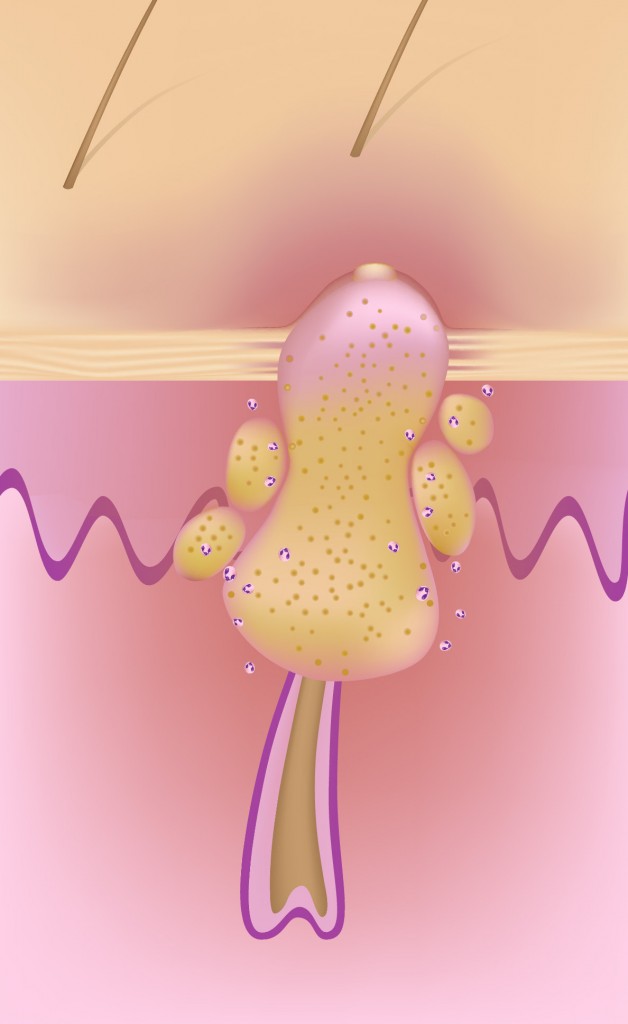
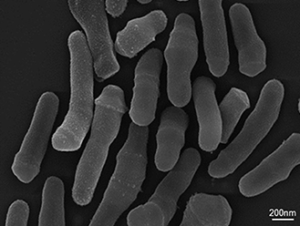
Skin commensals like Propionibacterium acnes (P. acnes), recently renamed Cutibacterium acnes (C. acnes), has long been implicated in the pathogenesis of acne, along with the other major patho-physiological factors of increased seborrhea, hyperkeratinization of the pilo-sebaceous unit and inflammation.
There are several preclinical mouse models of acne that utilize Cutibacterium acnes.
These models can help researchers understand the pathogenesis of acne and evaluate potential treatments.
This model is used as routine model at Imavita for R&D services purpose.
Protocol summary
- C. acnes culture until reaching log phase growth.
- Inoculum preparation and control.
- Mice details: Immunocompetent strain in SPF facility A1 or A2.
- Induction: C. acnes inoculum intradermal injection (ears).
- Treatments administration: test drugs via oral, IP, IV, SC or topical routes.
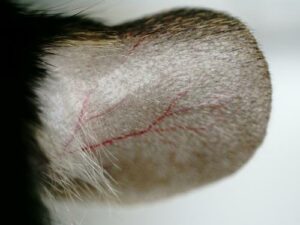
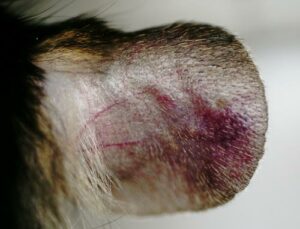
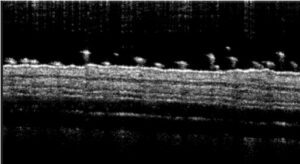
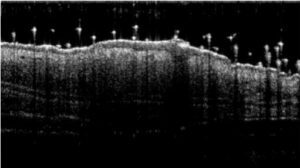
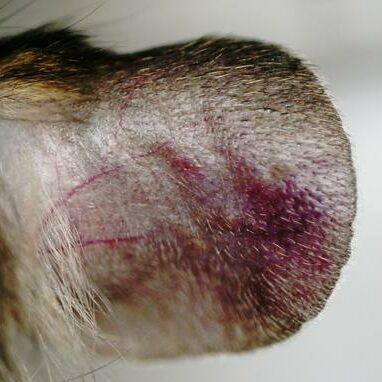
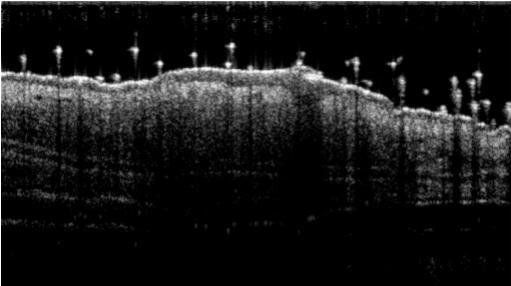
- Monitoring for maximum 5 days: bodyweights, daily calipering (ears thickness), visual macroscopic scoring (erythema, scaling), SkinColor® (erythema), digital pictures, OCT (epidermis/dermis thickness).
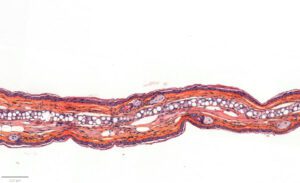
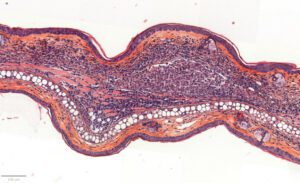
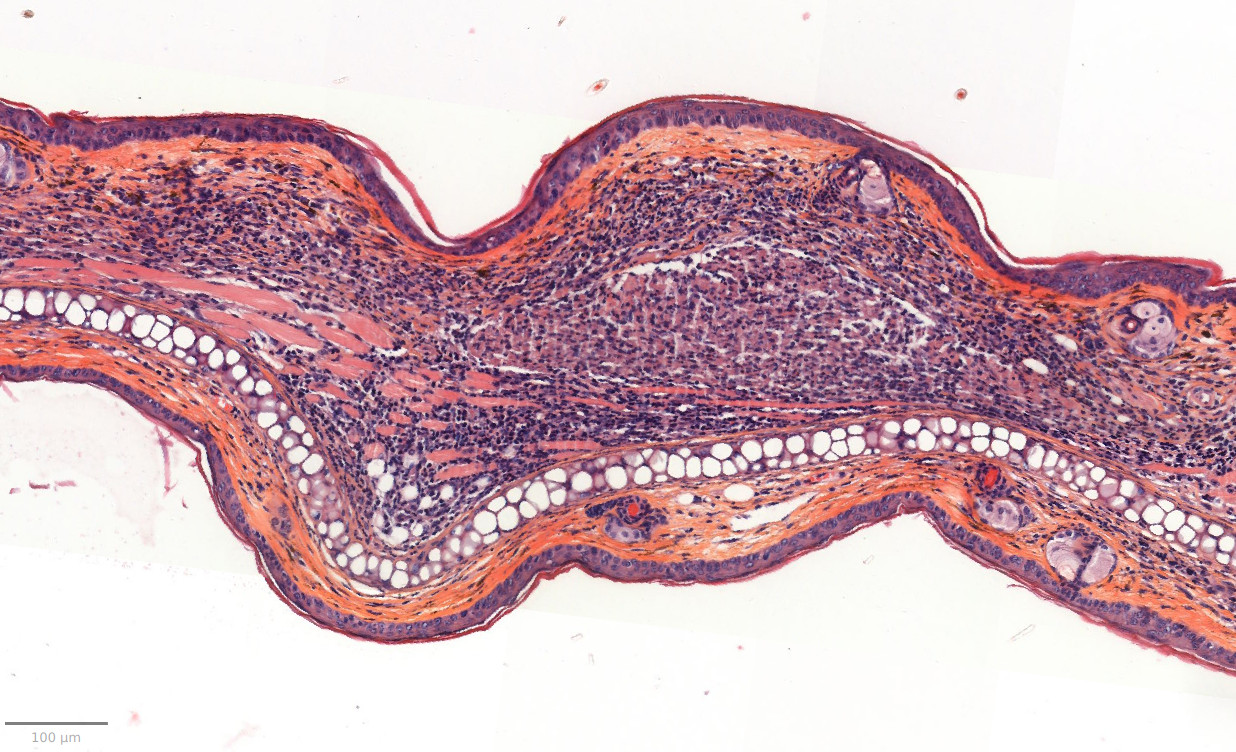
- Evaluation of inflammation: histology (HES), genes expression (qPCR) and cytokines levels (multiplexed ELISA) in ears.
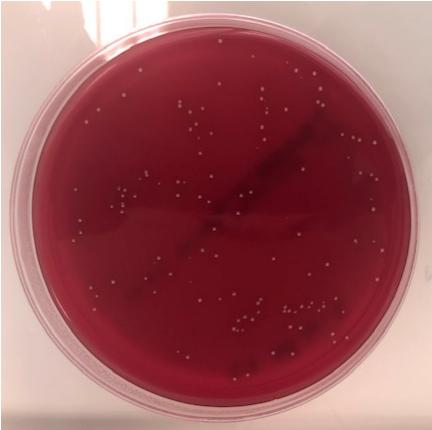
- Bacteriology / bacterial burden in tissues (CFU counting).
Typical results
Cutibacterium acnes C. acnes Acne Model / Detailed Protocol
01 - Bacteria strain:
– Propionibacterium acnes (P. acnes), renamed Cutibacterium acnes (C. acnes)
– Obtained from commercial sources
– Culture in anaerobic conditions until reaching log phase growth for induction
02 - Inoculum / Induction:
– After culture, concentration of bacteria in PBS
– Control of concentration (optical density / counting)
– Intradermal injection in ears under anesthesia
03 - Animal strain:
– Normal mice strain at induction
– Strain C57Bl6 (inbred) or CD1/Swiss (outbred)
– Health status: SPF at arrival in conventional/infectious zone / A2 facility after acclimatization about 1 week
– Immuno-competent at induction: age > 7 weeks
– Gender: Male or Female

04 - Application of dermatological pharmaceutical products:
– Ready to use formulations / Formulation screening on request
– Topical or Oral or IV or IP or SC routes
– Treatment duration: up to 5 days
– Reference drugs: Anti-inflammatory drugs (corticoid or specific mAb)
05 - Evaluation of in vivo clinical macroscopic signs:
06 - In vivo imaging:
07 - Ex vivo evaluations:
– Bacteriology for confirmation of presence of C.acnes and quantification in tissue homogenates
– Genes expression by qPCR on tissue extracts (i.e. IL1A / IL1B / MMP9 / TNFa / TLR2 / …)
– Cytokines assay using multiplexed ELISA (i.e. IL18 / IL1B / TNFa / IL6 / CXCL1 / …)
– Histology HES (Hemalun-Eosin-Safran) after paraffin inclusion with the following histomorphometrics analysis:
> Epidermis thickness measurement
> Dermis inflammation (cellularity)
> Dermis inflammation (tickness) +250-300%
> Other noticeable observations (parakeratosis, neutrophilic abscess, …)
Other ex vivo imaging techniques of interest:
– Specific stainings and imaging IHC or IF on request (in partnership)
– Specific imaging MALDI/MS on request (in partnership)
Cutibacterium acnes C. acnes Acne Model / Conclusion
This mouse model is a 1st intention model, fast and reproducible validated, with anti-inflammatory compounds as reference drug.
8 to 10 animals per group are generally sufficient to underline anti-acne effect of new therapeutics (based on difference to underline about 25 to 30%).
By using this model, researchers can gain a better understanding of the complex interactions between C. acnes and the host immune system, and investigate potential anti-acne therapies.
However, it’s important to note that preclinical mouse models are only approximations of the human condition and may not completely reflect the human specificity.
Cautions to be taken on this model:
Does PK of the test drug need to be evaluated first?
Drug pharmacokinetics / ADME / transcutaneous passage should be known to optimize drug dosing whatever the route. This is recommended, but not required.
Use of previously untested excipients should be avoided as they could cause false positive, false negative or local irritation. In any case, excipient negative group will be included.
Fomulation physico-chemistry should be well known (pH, osmolarity, etc…) as these factors can impact efficacy testing.
Project duration and follow-up:
Approximated duration 35 days.
for in vivo experiment until draft report for in vivo.
- Protocol setup and approval / 1-2 weeks
- Bacteria culture and in vivo experimentation / 2 weeks
- In vivo results / 1 week
- Draft report 1st version / 2 weeks
- Additional optional qPCR genes expression analysis / 1-2 weeks
- Additional optional proteins analysis using multiplexed ELISA / 1-2 weeks
- Additional optional histology / 6-7 weeks
Do not hesitate to contact us if you need more information or a quotation on this model.
Bibliography / Publications of interest / Cutibacterium acnes C. acnes Acne Model
1. De Young, L. M., Young, J. M., Ballaron, S. J., Spires, D. a. & Puhvel, S. M. Intradermal injection of P. acne: A model of inflammation relevant to acne. J. Invest Dermatol 83, 394–398 (1984).
2. Watson, J. D. Acne and related disorders. Br. J. Plast. Surg. 43, 393 (1990).
3. Nakatsuji, T., Liu, Y. T., Huang, C. P., Gallo, R. L. & Huang, C. M. Antibodies elicited by inactivated Propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: Relevance to therapy for acne vulgaris. J. Invest. Dermatol. 128, 2451–2457 (2008).
4. Isard, O. et al. Cutaneous induction of corticotropin releasing hormone by Propionibacterium acnes extracts. Dermatoendocrinol. 1, 96–9 (2009).
5. Scholz, C. F. P. & Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus propionibacterium to the proposed novel genera acidipropionibacterium gen. Nov., cutibacterium gen. nov. and pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 66, 4422–4432 (2016).
6. Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatology Venereol. 31, 8–12 (2017).
7. Nguyen, C. T., Sah, S. K. & Kim, T. Y. Inhibitory effects of superoxide dismutase 3 on Propionibacterium acnes-induced skin inflammation. Sci. Rep. 8, 1–12 (2018).
8. Kolar, S. L. et al. Propionibacterium acnes-induced immunopathology correlates with health and disease association. JCI insight 4, (2019).
9. Corvec, S., Dagnelie, M. A., Khammari, A. & Dréno, B. Taxonomy and phylogeny of Cutibacterium (formerly Propionibacterium) acnes in inflammatory skin diseases. Ann. Dermatol. Venereol. 146, 26–30 (2019).